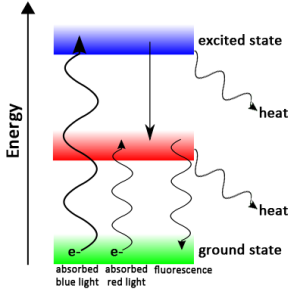
The Excited State of a Chlorophyll Molecule
When a photon of light hits chlorophyll, the electrons becomes excited and jump to a higher (more energetic) electron shell. If the chlorophyll is isolated, the electron quickly returns to is original state, releasing heat and fluorescence. A banana skin fluoresces due to excitation by different wavelengths of light: The upper left quadrant shows the unexcited banana skin, the upper right quadrant shows blue fluorescence due to UV excitation, the lower left quadrant shows green fluorescence due to blue excitation, and the lower right quadrant shows red fluorescence due to green excitation.
Green plants, algae, and many bacteria have similar chemical reaction centers, where light-harvesting molecules like chlorophylls are found. These reaction centers are part of larger complexes known as photosystem I and photosystem II, which capture the energy of light and make that energy usable. The structure of each light-absorbing pigment determines the wavelengths of light that can be absorbed.
Chlorophyll absorbs light most effectively from the blue portion of the electromagnetic spectrum, followed by the red portion. However, it is a poor absorber of green and near-green portions of the spectrum, hence the green color of chlorophyll-containing tissues. The double bonds of chlorophyll’s porphyrin ring, as well as the double bonds along the hydrocarbon chain connecting the two benzene rings in beta-carotene, contain freely moving electrons. These moving electrons can be hit, excited, and captured by photons of sunlight.
Photons are small bundles of energy that make up light. When a photon of the right wavelength (i.e., the right amount of energy) hits an electron, the electron becomes excited and jumps to a higher, unstable energy level (Figure 1). Pairs of excited electrons tend to be donated; that is, they pass through a series of electron donors (an electron transport chain), losing energy along the way. The excited electrons quickly return to their ground state (normal or lowest energy state), but the energy they produce in the process is ultimately trapped in new molecules (in this case, ATP and NADPH).
In summary, as light-absorbing molecules like chlorophylls and carotenoids receive sunlight, their electrons are constantly excited, and electron transport chains continually capture these excited electrons to form new, energy-containing molecules. This is the photo part of photosynthesis. The captured energy can then be used later in the synthesis part of the process, when sugars are made.
Materials Scientists Build Chlorophyll-Based Phototransistor
Coat a layer of graphene with chlorophyll and you get a remarkably sensitive light-activated switch, say physicists.
Chlorophyll is one of the most efficient light-absorbing materials known to science. It also happens to be remarkably stable and abundant beyond imagination.
Researchers studying ways to capture and exploit photons view the amazing capabilities of chlorophyll with green-eyed wonder. Indeed, they would dearly love to copy this extraordinary light-gathering ability.
But despite decades of research, artificial light-gathering systems are no match for their chlorophyll-based competitors. Not by any means.
But there may be a quick way of catching up. Instead of designing and making artificial materials that attempt to copy chlorophyll, researchers have begun to think about using this naturally-occurring material itself and attempting to bend it to their will.
Today, Shao-Yu Chen at the Institute of Atomic and Molecular Sciences in Taiwan and a few buddies reveal how they have incorporated chlorophyll into graphene transistors to make light-activated switches.
The new phototransistor design is relatively simple. It consists of two silver electrodes connected by a sheet of graphene. The graphene is then covered by a layer of chlorophyll using a method known as drop casting. This involves placing a drop of liquid containing chlorophyll on top of the graphene and letting it evaporate. This coats the graphene with a layer of chlorophyll.
This layer has a significant influence on the electronic characteristics of the device. When a voltage is set up between the silver electrodes, relatively little current flows. However, when the chlorophyll is zapped by light of certain frequencies, the current increases dramatically.
That’s because the light causes chlorophyll to release electrons into the graphene and this increases the current that flows. In fact, Shao-Yu and co say that every photon that the chlorophyll absorbs increases the current by about a million electrons.
That’s an interesting proof-of-principle device showing that chlorophyll can be incorporated into a graphene phototransistor relatively easily .
But this is still a crude device that will need significant optimisation before it might find any kind of real world application. Indeed, a crucial question is whether there are better ways to make graphene-based devices sensitive to light, for example with quantum dots or with other light-sensitive molecules.
What will drive the interest of materials scientists is the fantastic light-gathering capabilities of natural systems based on chlorophyll. Clearly, there is enormous potential here, if only they can find a way to unlock it.
Ref: arxiv.org/abs/1306.3015: Biologically Inspired Graphene-Chlorophyll Phototransistors